University of Toronto Engineering Team Reverse Engineers the Heart
The team built a bioartificial left ventricle, a lab-grown model made with living heart cells and capable of pumping fluid inside a tube, according to the University of Toronto.

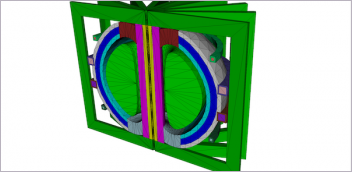
This model heart ventricle, made with real living heart cells, beats strongly enough to pump fluid inside a tube. It can be used to study heart disease and test potential therapies, without need for invasive surgery. Image courtesy of Sargol Okhovatian.
Latest News
July 12, 2022
University of Toronto Engineering researchers have grown a small-scale model of a human left heart ventricle in the lab. The bioartificial tissue construct is made with living heart cells and beats strongly enough to pump fluid inside a bioreactor, according to researchers.
The new lab-grown model could offer researchers a new way to study a range of heart diseases and conditions, and test potential therapies.
“With our model, we can measure ejection volume—how much fluid gets pushed out each time the ventricle contracts—as well as the pressure of that fluid,” says Sargol Okhovatian. “Both of these were nearly impossible to get with previous models.”
Okhovatian and Mohammad Hossein Mohammadi are co-lead authors on a new paper in Advanced Biology that describes the model they designed. Their multidisciplinary team was led by Professor Milica Radisic, senior author of the paper.
All three researchers are members of the Centre for Research and Applications in Fluidic Technologies (CRAFT). A partnership between Canada’s National Research Council and the University of Toronto, CRAFT is home to experts who design, build and test miniaturized devices to control fluid flow at the micron scale.
“The unique facilities we have at CRAFT enable us to create sophisticated organ-on-a-chip models like this one,” says Radisic.
“With these models, we can study not only cell function, but tissue function and organ function, all without the need for invasive surgery or animal experimentation. We can also use them to screen large libraries of drug candidate molecules for positive or negative effects.”
Many challenges facing tissue engineers relate to geometry: while it’s easy to grow human cells in two dimensions—for example, in a flat petri dish—the results don’t look much like real tissue or organs as they would appear in the human body.
To move into three dimensions, Radisic and her team use tiny scaffolds made from biocompatible polymers. The scaffolds, which are often patterned with grooves or mesh-like structures, are seeded with heart muscle cells and left to grow in a liquid medium.
Over time, the living cells grow together, forming a tissue. The underlying shape or pattern of the scaffold encourages the growing cells to align or stretch in a particular direction. Electrical pulses can even be used to control how fast they beat.
For the bioartificial left ventricle, Okhovatian and Mohammadi created a scaffold shaped like a flat sheet of three mesh-like panels. After seeding the scaffold with cells and allowing them to grow for about a week, the researchers rolled the sheet around a hollow polymer shaft called a mandrel.
The resulting tube is composed of three overlapping layers of heart cells that beat in unison, pumping fluid out of the hole at the end. The inner diameter of the tube is 0.5 mm and its height is about 1 mm, making it the size of the ventricle in a human fetus at about the 19th week of gestation.
Sources: Press materials received from the company and additional information gleaned from the company’s website.
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
DE’s editors contribute news and new product announcements to Digital Engineering.
Press releases may be sent to them via DE-Editors@digitaleng.news.