Axial3D Scores FDA Clearance
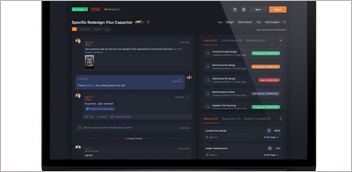
Axial3D INSIGHT automates the process of converting 2D DICOM images into 3D print-ready files.

Axial insight helps medical device makers accelerate patient-specific programs. Image Courtesy of Axial3D
Latest News
August 7, 2023
Patient-specific devices are the next frontier in personalized medical care, with 3D printing at the forefront to allow hospitals and medical device makers to capitalize on the emerging trend.
Axial3D, a leader in medical segmentation and 3D printing solutions, has achieved a critical step that will help the medical industry inch closer to its goal of making 3D printed devices widely available to improve patient outcomes. The company has received FDA clearance for its automated, AI-driven cloud-based segmentation software platform used to accelerate 3D printing of custom tools and solutions for orthopedic trauma, orthopedic, maxillofacial, and cardiovascular applications.
The 510(k) cleared software employs AI and the scalability of the cloud to automate the conversion of patients’ DICOM images from CT and MRI scans into accurate 3D visualizations and 3D print-ready files, 3D mesh files, and even 3D printed anatomical models made with Stratasys print technology.
By automating the traditionally arduous and time-consuming process associated with manual or even semi-manual segmentation, Axial3D opens the door for healthcare providers and medical device makers to handle more patient-specific applications in shorter time frames and with significantly less capital expenditures. The software enables more patient data to be processed, accelerating the development of 3D printed patient-specific programs for creating surgical plans, models, and surgical guides.
“With Axial3D INSIGHT, they can move from today’s world, which is a serialized manufacturing workflow, to a patient-specific workflow, allowing them to greatly enhance the standard of care for patients and the development they can bring to their medical products,” says Daniel Crawford, the founder and chief strategy office at Axial3D.
Stratasys invested $10 million in Axial3D in late 2022 and fostered a partnership to bring a joint solution to market for both healthcare providers and medical device makers. A range of Stratasys printers and materials have been validated and FDA 510(k) cleared with Axial3D INSIGHT to produce anatomic models for pre-operative surgical planning and for diagnostic use across multiple specialties. Stratasys estimates the opportunity for medical 3D printing at approximately $2.8 billion.
In this webinar, Axial3D’s Daniel Crawford joins a panel discussion on the state of 3D printing in medicine.
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Beth Stackpole is a contributing editor to Digital Engineering. Send e-mail about this article to DE-Editors@digitaleng.news.
Follow DE